Conformational ensembles
Contents
Conformational ensembles#
For many larger molecules a single structure approach for computing properties is insufficient since several relevant structures are populated at room temperature.
In this tutorial we will use the conformer–rotamer ensemble search tool (CREST) for generating a conformational ensemble.1
An interface from CREST to DFTB+ is currently under development, therefore we will use the xtb program package2 to provide access to the extended tight binding (xTB) Hamiltonian.
Setting up CREST#
First, ensure that the crest and the xtb binary are found in your PATH.
You can install the programs with the mamba package manager using
mamba create -n conf crest xtb 'libblas=*=*mkl*'
mamba activate conf
For optimal performance pin the BLAS library to the Intel MKL if available on your platform.
Environment effects on side-chains#
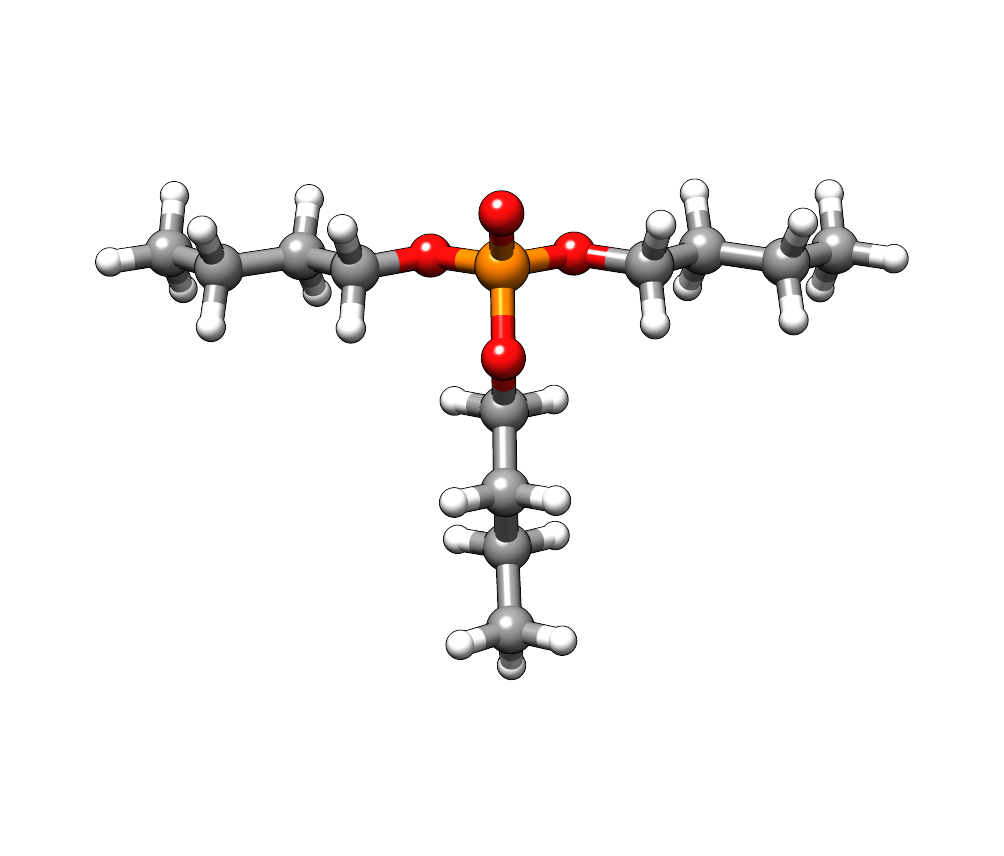
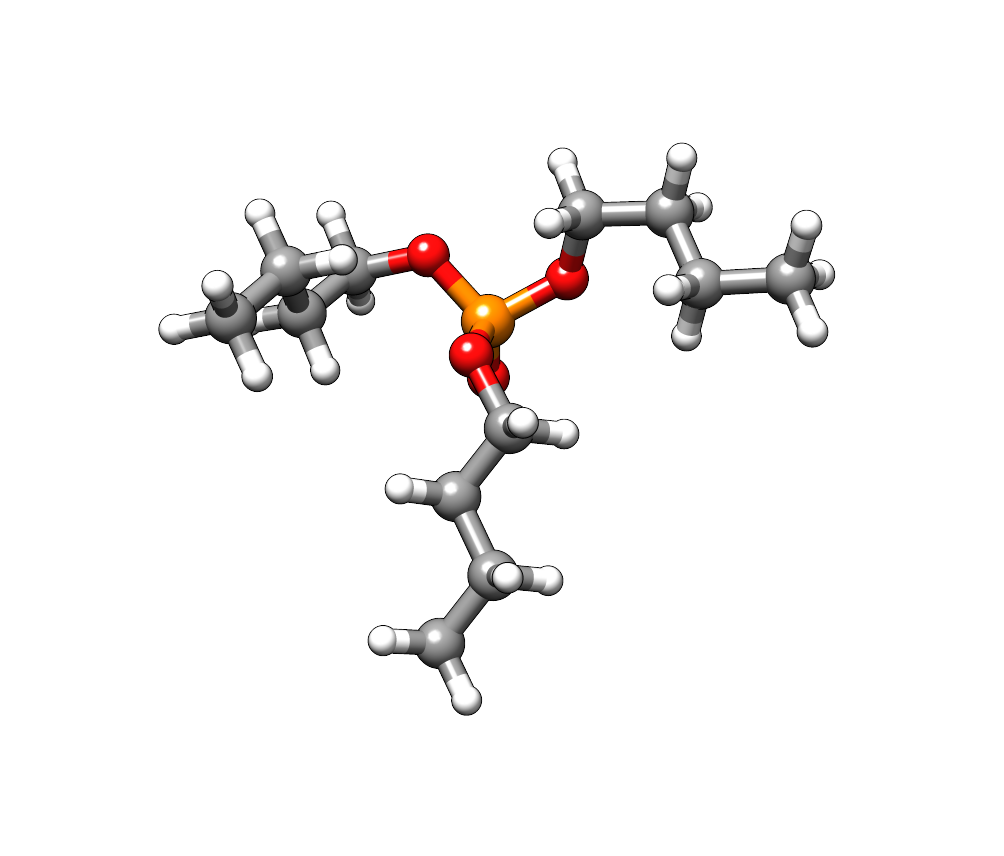
For the compound tributyl phosphate we download the structure from the PubChem database. The three butyl chains provide conformational flexibility and the just retrieved structure might not describe the energetically most favorable conformation.
44
31357
P -0.01860000000000 0.96240000000000 0.86270000000000
O 1.24730000000000 1.23990000000000 -0.10990000000000
O -1.28770000000000 1.20560000000000 -0.11480000000000
O 0.00250000000000 -0.63950000000000 1.10700000000000
O -0.03210000000000 1.78240000000000 2.12160000000000
C 3.56530000000000 1.33540000000000 -0.69040000000000
C -3.60530000000000 1.23840000000000 -0.70410000000000
C 0.03500000000000 -2.95840000000000 0.52240000000000
C 2.55080000000000 1.00760000000000 0.39580000000000
C -2.58640000000000 0.93820000000000 0.38600000000000
C 0.01670000000000 -1.52800000000000 0.00260000000000
C 4.99570000000000 1.09180000000000 -0.21750000000000
C -5.03260000000000 0.98640000000000 -0.22620000000000
C 0.06690000000000 -3.97200000000000 -0.61800000000000
C 6.00320000000000 1.37910000000000 -1.31920000000000
C -6.04940000000000 1.32860000000000 -1.30350000000000
C 0.12870000000000 -5.39790000000000 -0.09450000000000
H 3.35100000000000 0.72850000000000 -1.57910000000000
H 3.44050000000000 2.37950000000000 -1.00350000000000
H -3.49160000000000 2.28050000000000 -1.02810000000000
H -3.38590000000000 0.62470000000000 -1.58670000000000
H 0.90630000000000 -3.09410000000000 1.17540000000000
H -0.84420000000000 -3.12700000000000 1.15680000000000
H 2.64090000000000 -0.03930000000000 0.70250000000000
H 2.71020000000000 1.64300000000000 1.27340000000000
H -2.76500000000000 1.56970000000000 1.26270000000000
H -2.65050000000000 -0.11060000000000 0.69310000000000
H 0.90370000000000 -1.33520000000000 -0.60870000000000
H -0.87220000000000 -1.36000000000000 -0.61330000000000
H 5.10860000000000 0.05110000000000 0.10790000000000
H 5.21510000000000 1.72800000000000 0.64780000000000
H -5.23930000000000 1.58980000000000 0.66540000000000
H -5.14910000000000 -0.06540000000000 0.05960000000000
H 0.93850000000000 -3.78720000000000 -1.25660000000000
H -0.82500000000000 -3.85540000000000 -1.24460000000000
H 5.83130000000000 0.73360000000000 -2.18640000000000
H 5.93810000000000 2.42120000000000 -1.64800000000000
H 7.02080000000000 1.19960000000000 -0.95850000000000
H -5.98120000000000 2.38380000000000 -1.58660000000000
H -5.89010000000000 0.72100000000000 -2.20000000000000
H -7.06450000000000 1.13910000000000 -0.94050000000000
H 1.02870000000000 -5.55600000000000 0.50830000000000
H -0.74450000000000 -5.62650000000000 0.52480000000000
H 0.14930000000000 -6.10710000000000 -0.92790000000000
To start a calculation with CREST we create a new directory and add the initial structure.
❯ tree .
.
└── pubchem.xyz
For the conformer search itself we will use the program defaults which will use the GFN2-xTB method for the sampling
❯ crest pubchem.xyz -T $(nproc) -mquick | tee crest.out
Note
The runtime for sampling the conformer ensemble depends mainly on the flexibility of the system.
The conformer search can be parallelized on a shared memory system using the -T option to speed-up the calculation.
Additionally, the sampling quality can be changed by using the -quick, -squick, or -mquick.
We will use -squick option here which is the faster than a full search, but might also result in potentially incomplete ensemble.
For production runs try to avoid reducing the sampling quality.
After the calculation has finished we check the files produced by CREST
❯ tree .
.
├── coord
├── coord.original
├── cre_members
├── crest_best.xyz
├── crest_conformers.xyz
├── crest.energies
├── crest.out
├── crest_rotamers.xyz
├── gfnff_adjacency
├── gfnff_charges
├── gfnff_topo
├── hosts_file
├── job
├── pubchem.xyz
├── struc.xyz
└── wbo
crest_best.xyz:Contains the lowest conformer of the ensemble
crest_conformers.xyz:Contains the whole conformer ensemble ordered energetically. A viewer, like molden, can be used to inspect the ensemble and the relative conformational energies (in kcal/mol).
crest.energies:Contains the relative conformer energies in kcal/mol for plotting.
Exercise
Compare the found ensemble with the energetically most favorable conformer found by DFT.
44
P 0.04979860695690 0.44711451464043 -1.00582935088785
O -1.01828570201577 1.61992081924505 -1.08204989992478
O 1.44541819964837 1.20259350968357 -0.93004646393268
O -0.23116449461962 -0.19092086100911 0.43348057584036
O 0.04989811059549 -0.52939857862507 -2.13193727489898
C -3.22595849860852 0.57569749292020 -0.63048371070980
C 3.17125059294208 2.26919500606264 0.37793012664706
C -0.49671006005934 -2.62276723852782 0.51752296451488
C -2.37291235771157 1.37377084394425 -1.59520657808180
C 1.69082363056085 2.25168490238616 0.06491515604763
C 0.42423494323872 -1.45148539577987 0.79538479831033
C -3.40028045343485 1.23959706437998 0.73452517731784
C 3.66251230954912 1.00475672173489 1.08259311966198
C 0.14365149763826 -3.95555023713892 0.91118196598503
C -4.25721021459863 0.39844726585583 1.67794675785264
C 5.14863386178168 1.07197599507234 1.42894858037114
C -0.77275034832669 -5.13994447582840 0.60668692357785
H -4.20593926439767 0.44583117295978 -1.11148258102692
H -2.80524727780564 -0.43169945847273 -0.50895563008287
H 3.73396375795546 2.42972141752258 -0.55124157858279
H 3.35560124098091 3.14584756668454 1.01426453778633
H -1.43320670149735 -2.47989437852294 1.07245708104755
H -0.74649279399252 -2.63205125963662 -0.55075018369840
H -2.28435686339904 0.87349896173282 -2.56570622164894
H -2.77764831943878 2.37852774636938 -1.74559624331743
H 1.10045874685666 2.04089452960540 0.96551158716685
H 1.35623984857714 3.19828298483888 -0.37219697034341
H 0.64786384718387 -1.35765077566991 1.86259993322524
H 1.37041338393201 -1.54864147188351 0.24797788513280
H -3.85688415770214 2.22927316800735 0.59672718397360
H -2.41375306800835 1.40599090456599 1.18221915186467
H 3.47264091184327 0.13643034401191 0.44003684602247
H 3.07393423247992 0.85408789825825 1.99820770460226
H 0.39073192854148 -3.94380191866237 1.98125890567555
H 1.09263532764405 -4.07715111779153 0.37207018493030
H -3.79637849612223 -0.58020445552568 1.85370039309811
H -4.38030266458304 0.88988483294634 2.64812230808074
H -5.25489295914165 0.22661390262107 1.25834559004993
H 5.47806835596330 0.16138801918681 1.93896808119261
H 5.75695498422814 1.18910728560937 0.52530220715744
H 5.36133224641826 1.92201274229434 2.08696405672930
H -1.00459785333360 -5.18748938484018 -0.46299441722211
H -0.30604962300523 -6.08751382128645 0.89294337567665
H -1.71987305808669 -5.05272568826932 1.15031517963084
The DFT ensemble was generated in an implicit solvent, namely octanol. Check the influence of including implicit solvation in the generation of the ensemble. The help page of CREST can give you insights which options are required for activating the implicit solvation model.
Summary#
You learned…
to find the energetically most favorable conformer of a compound
sample a conformational ensemble and compare it with a reference
include environment effects like solvation in your conformer search
Literature#
- 1
Philipp Pracht, Fabian Bohle, and Stefan Grimme. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys., 22:7169–7192, 2020. doi:10.1039/C9CP06869D.
- 2
Christoph Bannwarth, Eike Caldeweyher, Sebastian Ehlert, Andreas Hansen, Philipp Pracht, Jakob Seibert, Sebastian Spicher, and Stefan Grimme. Extended tight‐binding quantum chemistry methods. WIREs Comput. Mol. Sci., 11:e1493, 2021. doi:10.1002/wcms.1493.